When Fighting Disease Becomes a Family Affair
Parents and relatives of people afflicted with genetic diseases take a leading role in advancing medical research




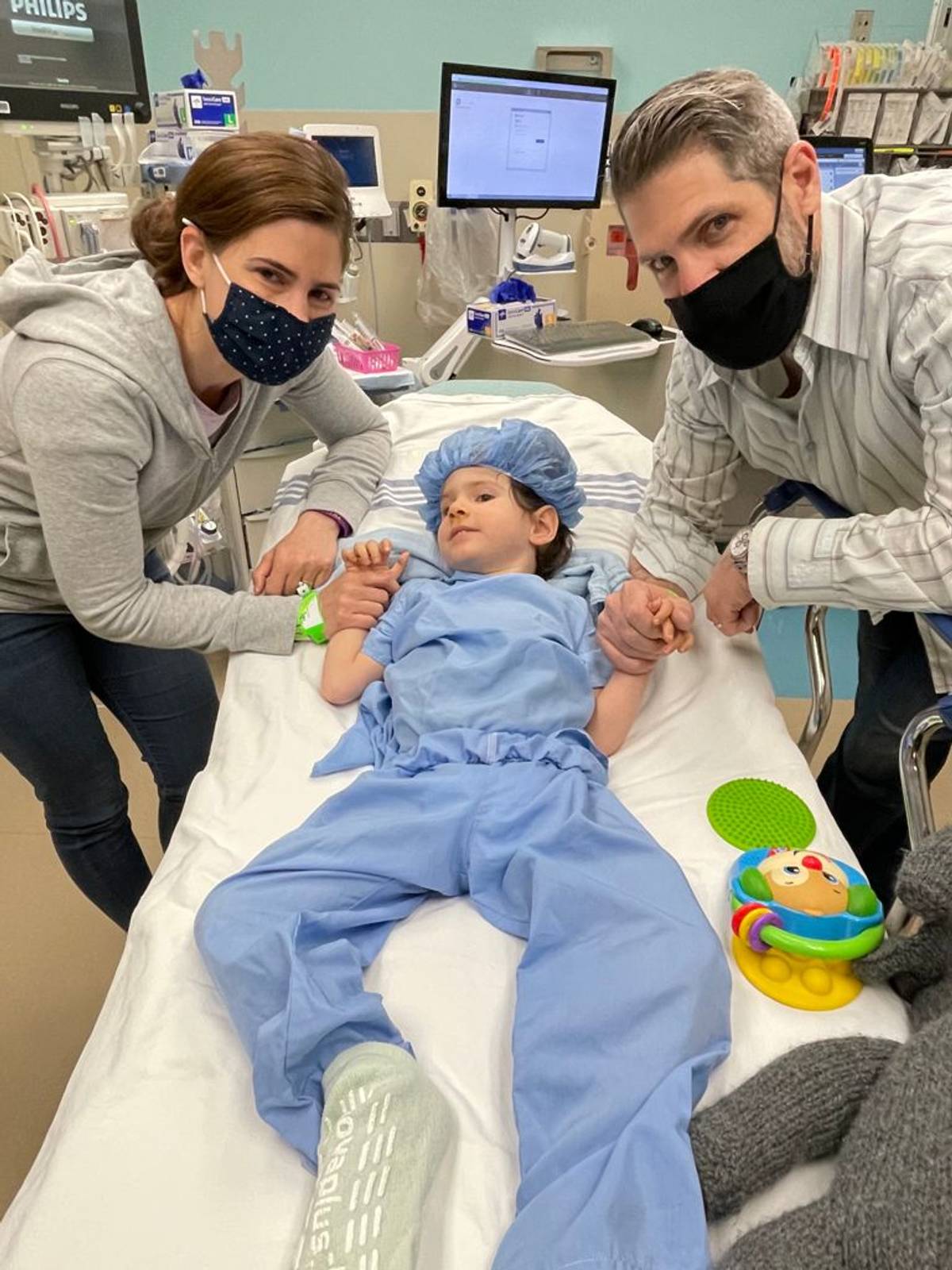
When a doctor diagnosed Jennie Rosenblum Landsman’s two baby boys with a degenerative, fatal neurological disease, he told her to go home and make them comfortable because there was nothing else to do. He added that she should not waste her time looking for false hope online.
Landsman ignored the doctor, and within three weeks, after hours of online reading, she connected with a researcher focusing on gene therapy for Canavan disease, the devastating genetic condition her children have—one of many genetic diseases that disproportionately affect Ashkenazi Jews. Those with Canavan don’t make enough of a protein called myelin, which is crucial for brain and nerve function; they never walk or talk, eventually lose the ability to swallow, and usually die by the age of 10.
Landsman recalled: “We went home broken after the diagnosis,” which happened when her son Benny was 13 months, and Josh was 2 weeks old. “But I started scouring the internet, staying up all night and reading everything I could, writing to other parents and to researchers. Some answered me, and some didn’t.”
One of those who answered, calling back within 15 minutes, was Paola Leone, director of the cell and gene therapy center at Rowan University, who had treated 13 Canavan patients with gene therapy back in 2001, with pretty disappointing results; it had not caused harm, but it didn’t help much either, mainly because the virus used to deliver the gene therapy couldn’t penetrate the brain and nervous system long term. But just before Landsman reached out to her, Leone had developed a new way to deliver the therapy to repair the defective gene directly into the white matter of the brain; it had worked in rats, but it would cost $1.5 million to make just a few human doses.
Landsman, a martial arts and yoga teacher, said she would raise the money so her kids could try the treatment. Four years and $5 million later, Benny and Josh were treated this spring. She says they have already shown some improvements, including appearing more alert, and having more coordinated hand movements and quicker use of special computers to communicate.
“Now, instead of just waiting for them to lose more abilities, we are looking for new things they can do,” Landsman said. “And that’s incredible.”
While almost unbelievable, the path Landsman took—raising money to create a treatment for her own children—is an increasingly common way to deal with rare genetic diseases, including those that have affected Jewish communities at higher-than-average rates. While families of those suffering from diseases have long banded together to raise awareness and money, the advancements in genetic science and the internet have now made it possible for parents and others to start new tailored projects and see some results—or even a treatment—in just a few years. With the world’s first FDA-approved gene therapies coming out in the past few years, including for spinal muscular atrophy and a specific type of blindness, people have hope that similar treatments could be available for other diseases, too. There were currently more than 400 clinical trials underway for gene therapies in 2020.
“Families and patients are playing a bigger role in rare disease research,” said Ed Neilan, chief medical and scientific officer at the National Organization for Rare Disorders, a nonprofit advocating for those with rare conditions. “Patients and families are recruiting and funding researchers to develop specific gene therapies. Of course, a couple of decades ago that would have seemed impossible, but today there are more and more examples of drugs—including a few gene therapies—developed for very rare diseases, and there is also more awareness of gene therapies. Some efforts of this sort have been started by wealthy parents with backgrounds in finance and big business, sometimes by starting a new biotech company with a disease-specific mission. More often, such efforts have been started with more modest means.”
When Scott Reich’s son Eli was diagnosed with a genetic brain disease called FOXG1 syndrome in 2019, doctors gave him advice similar to that Landsman received: There is nothing to do, just go home and love your son.
“That just wasn’t an acceptable answer for us,” said Reich, a lawyer from Long Island. He and his wife began reading everything they could about the disease, reaching out to researchers around the world who were working on it, and recruiting new scientists to the topic.
“We had to become scientists overnight,” Reich said. They ended up connecting researchers around the world with each other, and founding a nonprofit organization called Believe in a Cure to raise more funds and awareness. Now, there are several lab and animal studies underway, including one at Tel Aviv University, seeking to figure out if any existing drugs can treat the disease—which causes physical disability, severe cognitive impairment, and seizures, and may affect Jewish populations more frequently.
“Without these families pushing us, the research would be much slower,” said Eddy Pichinuk, a molecular cell biologist at Tel Aviv University’s Blavatnik Center for Drug Discovery, which is working with Reich and other families on finding drugs for several different rare diseases. “They keep asking us questions, and connecting us to other researchers so we can collaborate better, it definitely helps.” The center hears daily from families of patients with rare disorders looking for researchers who can help.
It’s not just parents who are dedicating their lives to fund research, but also other family members and sometimes patients themselves. Several patients grappling with adult polyglucosan body disease and their family members were among those who worked hundreds of hours to secure a grant last year from the Chan Zuckerberg Initiative’s Rare as One Project , which will help it fund collaboration between researchers, and eventually, hopefully, pave the way toward clinical trials.
Clinical trials are the dream for all who suffer with APBD, which also disproportionately affects Jews. The disorder involves progressive neurodegeneration, with symptoms of nerve damage and bladder dysfunction usually beginning in the mid-30s.
“Finally we have light at the end of the tunnel,” said Emil Weiss, co-president of the Adult Polyglucosan Body Disease Research Foundation. Emil and his brother Michael struggle with the ravaging effects of APBD, as did their brother Gregory, who started the foundation in 2005.
Jeff Levenson, co-president of the foundation, said: “We could not have come this far without the help of the patients in our community; these people are really our superheroes. As a result of their donations and their engagement, our work to find treatments and cures is progressing rapidly.” Levenson lost his father and uncle to APBD a few years before he learned about the foundation and joined its early work.
Recently the U.S. Food and Drug Administration, which must approve treatments, has made more efforts to work with patients and their families, including them in meetings and hearings throughout the drug-approval process.
“These initiatives are often focused primarily on encouraging the choice of clinical trial endpoints that are relevant and meaningful to the affected families,” Neilan said.
But for those families who lead such initiatives, it is a life-consuming quest to learn the science behind a disease, find the relevant researchers, and raise funds to enable research while caring for sick or disabled children.
“It’s really difficult to separate our work on the foundation from other aspects of life,” Reich said. “Actually there is no separation, and it’s all-consuming. When you are trying to save your kid’s life, there is no mountain you won’t climb.”
Landsman, who gave birth to two other healthy children since her sons were diagnosed, eventually closed her business because she did not have time for it. Working with doctors, FDA officials, and others to set up the gene therapy was more than a full-time job, she said. “If I had realized when I first talked to Dr. Leone how much money and work this was going to take, I probably would have given up,” she said. “I’m glad I didn’t really know anything back then, or we would not have gotten here.”
The advances in genetic testing are also crucial in the field of gene therapy and other possible treatments, experts say. Testing has drastically reduced the number of patients with some genetic diseases, especially in Jewish communities, due to parents who know they are carriers deciding not to conceive, opting to terminate affected pregnancies, or relying on IVF processes that only implant healthy fertilized eggs in women, said Estie Rose, a genetic counselor at JScreen, a nonprofit operating out of Emory University that supplies at-home testing kits to screen for Jewish genetic diseases, and counseling about the results. Testing is now much cheaper and more accessible, often requiring saliva rather than blood samples, Rose said.
“The Jewish community has taken ownership of its health and has had great success in the area of preventing genetic diseases,” she said.
But as gene therapy inches toward reality, testing is, perhaps, even more important because it allows parents to find out early on if they are carrying or have given birth to a child with a genetic disorder, and thus to start treatment as early as possible, said Shari Ungerleider, project coordinator at the Jewish Genetic Disease Consortium. In almost all cases, gene therapy appears most effective when given early, ideally before the onset of symptoms.
“Science has advanced more than we could ever have imagined,” Ungerleider said. “It is amazing to think of what could be done even in utero, but testing remains key so that people will know they need to start any available treatments.”
Before Landsman got pregnant with her first child, now 11, she completed a standard panel of reproductive genetic screening aimed at those with Ashkenazi heritage, was told she tested negative for everything, including Canavan, and gave birth to a healthy son. So when she decided to have more children, several years later with her current husband, she didn’t do any more testing.
“I thought everything was OK,” she said. When Benny was born in 2016, he was fine until he was about 6 months old, “when he just plateaued and stopped developing,” never learning to crawl or try to stand up.
“We spent the next six months going to multiple doctors, who could not figure out what was wrong,” she recalled. “I was not even thinking it was anything genetic.” Then when Benny was 13 months old and his baby brother Josh just a few weeks old, a doctor detected a sign in Benny’s urine, indicating a possibility of Canavan. Both boys ultimately were diagnosed with it.
Landsman also obtained her previous genetic testing results, and realized the Ashkenazi panel was never actually performed.
“It was a mistake by the lab, and also not noticed by my doctor,” she said.
Since then, it has been a race against time, even as science and gene therapies are advancing; it’s a common experience for those suffering from genetic diseases, which are often degenerative. While Emil Weiss is happy that clinical trials that he has worked so hard to advance may be on the horizon, he is aware they may not be beneficial to him and other older patients.
“Unfortunately my condition has worsened a lot and it’s an effort just to get through the day’s activities,” Weiss said. “I’m hopeful our work will help many other patients.”
A brighter future is also on the mind of Landsman, not only when she thinks of her own children, but when she thinks of the dozens of others, some much younger than her boys, who are scheduled to receive the gene therapy in the coming months. Even though the gene associated with Canavan occurs at higher frequencies among Ashkenazi Jews, most of these children are not Jewish “because Jewish people know to get tested, but others don’t necessarily know,” Landsman said. The same is now true for other diseases, including Tay-Sachs.
Shortly before leaving Dayton, Ohio, in July to return home after Josh was treated, Landsman met a girl from Italy who was about to undergo the same treatment that she had worked so hard to make a reality. That, she said, was an emotional experience: “Knowing that this had a bigger impact than just my family—there are no words for that.”
(Editor’s note: An earlier version of this story incorrectly indicated that there had not been any clinical trials for APBD.)
Sara Toth Stub is a Jerusalem-based American journalist who has written for The Wall Street Journal, Dow Jones Newswires, Associated Press, and other publications.